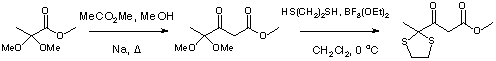|
Preparation of methyl 3,4-dioxopentanoate-4-ethylenedithioacetal; monoprotected 3,4-dioxo-ester SyntheticPage 204 (2002)
Submitted 8th Oct 2002, published 16th Oct 2002
Carlos Afonso
([email protected]),
A contribution from the Carlos A. M. Afonso, C. D. Maycock Group, New University of Lisbon, Portugal
Chemicals Used
Sodium (Merck), methyl pyruvate (Aldrich), anhydrous methyl acetate (distilled from P2O5), 1,2-ethanedithiol (Merck), boron trifluoride diethyl etherate (Aldrich, fresh distilled), anhydrous dichloromethane (distilled from P2O5), anhydrous methanol (distilled from Mg), trimethyl orthoformate (Merck), p-toluenesulfonic acid (Merck). Methyl 2,2-dimethoxypropanoate was prepared by slow distillation (40-50 oC, 7 h, 6-7 mL) of a reflux solution (70 oC) of methyl 2-oxo-propanoate (10.0 g, 97.95 mmol), trimethyl orthoformate (11.8 mL, 1.1 eq), anhydrous methanol (14.7 mL) and p-toluenesulfonic acid (0.24 g, 1.3 mol%) followed by distillation (78-80 oC/water aspirator pump) giving 10.96 g (75 %) of the product as a clear liquid.
Procedure
To a stirred solution of methyl 2,2-dimethoxypropanoate (10.89 g, 73.5 mmol), methyl acetate (10.8 mL, 136.0 mmol) and anhydrous methanol (0.1 mL) was added sodium pieces (< 5 mm, 2.1 g, 90.5 mmol) and the mixture was stirred at 60 oC for 16 h (orange solution). More methyl acetate (10.8 mL, 136.0 mmol) and sodium sodium pieces (< 5 mm, 2.1 g, 90.5 mmol) were added and stirring continued for a further 8 h. The reaction mixture was allowed to cool, ice added (40 g), acidified with hydrochloric acid 12 M (final pH of 6) and was extracted with diethyl ether (4 x 60 mL). The combined organic phase was dried (MgSO4), evaporated under vacumm and distilled (62-66 oC/0.3 mmHg) giving methyl 4,4-dimethoxy-3-oxopentanoate (7.98 g, 57 %) as a clear liquid.
The above methyl ester (6.41 g, 33.7 mmol) was added to a stirred cold (ice bath) solution of anhydrous dichloromethane (20 mL) under an argon atmosphere, followed by the dropwise addition (2 h) of a solution of boron trifluoride diethyl etherate (3.4 mL, 33.7 mmol) and 1,2-ethanedithiol (2.83 mL, 33.7 mmol) in anhydrous dichloromethane (14 mL) and stirring was continued for a further 1h. Aqueous saturated NaHCO3 (20 mL), was added followed by extration with dichloromethane (3 x 30 mL). The combined organic phases were dried (MgSO4), evaporated under vacuum and purified by flash chromatography (SiO2, eluent: petroleum ether 40/60 : ethyl acetate from 1:0 to 6:4) to give the title compound (5.57 g, 75 %), as colorless liquid, Rf = 0.50 (CHCl3).
Author's Comments
The first step has been carried out several times on scales of 60 to 70 mmol. In case of the second step none of the isomeric methyl 3,4-dioxopentanoate-3-ethylenedithioacetal was detected by 1H NMR from the crude reaction mixture. The two step procedure was applied to the preparation of ethyl 3,4-dioxopentanoate-4-ethylenedithioacetal (first step 50 %, second step 74%). The second step was also applied to the preparation of monodithioacetals from different 1,2-dicarbonyl compounds (7 examples). Due to the strong smell of thiols is strongly recommended to use a very well ventilated fumehood.
Data
1H NMR (60 MHz, CCl4), (aprox. of 4% of the enol form) d: 1.82 (3H, s, C-5 Me), 1.94 (s, C-5 Me of the enol), 3.35 (s, S(CH2)2S of the enol), 3.45 (s, S(CH2)2S), 3.70 (2H, s, C-2 CH2), 3.80 (3H, s, CO2CH3), 5.60 (s, C(2)H of the enol); IR (film): 2960, 2940, 2850, 1745, 1710, 1620, 1440, 1405, 1375, 1320, 1265, 1205, 1140, 1100, 1075, 1040, 1000, 955, 850 cm-1; MS (EI, m/e): 220 (M+), 189, 177, 147, 145, 133, 128; Acc. Mass. (EI) calcd for C8H12O3S2: 220.0227; observed: 220.0236.
Lead Reference
Afonso, C. A. M.; Barros, M. T.; Godinho, L. S.; Maycock, C. D. Synthesis 1991, 575.
Other References
The first step was based on original similar reported conditions: Bettesti, P.; Battesti, O.; Sélim, M. Bull. Soc. Chim. Fr. 1974, 2214.
For the second step see also: Theodora W. Greene, Peter G. m. Wuts, Protective Groups in Organic Synthesis, third edition, John Wiley & Sons, 1999, pp 333-336.
This page has been viewed approximately 552 times since records began.
Get CDX file (Choose "Open this file from its current location" TWICE)
SyntheticPagesTM are for use
exclusively by those with training and experience in synthetic chemistry. While contributors
are asked to identify hazards and to use methods designed to reduce risk, no formal hazard
or risk assessments are included, and these procedures must be conducted at one's own risk.
SyntheticPages, its editors, owners and associates do not warrant or guarantee the safety of
individuals using these procedures and hereby disclaim any liability for any injuries or damages
claimed to have resulted from or related in any way to the procedures herein. Copyright ©2003 Synthetic Pages
Comment on this page!
Registered users may add comments to other pages. If you have already registered, please
log in, otherwise you may
register here.
|